Wang 2020 Remdesivir
Remdesivir also GS-5734 is a monophosphoramidate prodrug of an adenosine analogue that has a broad antiviral spectrum including filoviruses paramyxoviruses pneumoviruses and coronaviruses12 13 In vitro remdesivir inhibits all human and animal coronaviruses tested to date including SARS-CoV-213 14 15 and has shown antiviral and clinical effects in animal. Mo Wang Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs Frontiers Science Center for Transformative Molecules School of Chemistry and Chemical Engineering Shanghai Jiao Tong University 800 Dongchuan Road Shanghai 200240 China.
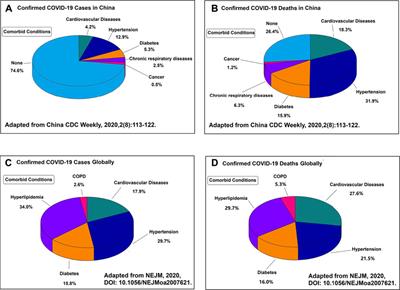
Frontiers Adverse Cardiovascular Effects Of Anti Covid 19 Drugs Pharmacology
A randomised double-blind placebo-controlled multicentre trial.
Wang 2020 remdesivir. To submit a request. Additionally 19 confirmed cases were identified in Hong Kong Macao and Taiwan and 39 imported cases were identified in Thailand Japan South Korea United States Vietnam. Cell Research 2020 30269271.
Wang Y Zhang D Du G et al. Remdesivir also GS-5734 is a monophosphoramidate prodrug of an adenosine analogue that has a broad antiviral spectrum including filoviruses paramyxoviruses pneumoviruses and coro na viruses. Cell Res 30 269271 2020.
This is a double blind placebo controlled trial from 10 hospitals in China. Patients were excluded if they had ASTALT derangement renal impairment pregnancy or breastfeeding anticipated discharge within 72 hours or were involved in another trial. The initial cases were linked to exposures in a seafood market in Wuhan.
In this Article a sentence has been added to the Acknowledgments as follows. Similar results were observed for remdesivir-treated SARS-CoV-2 Wang et al 2020. It is viewed as one of the worlds most promising treatments for COVID-19.
Lancet 2020 Apr 29. A randomised double-blind placebo-controlled multicentre trial. Remdesivir February 2020 Remdesivir RDV GS-5734 is an adenosine nucleotide analog that is active against a wide variety of single stranded RNA viruses 1 such as emerging and zoonotic coronaviruses 2 including the novel coronavirus 2019-nCoV 3 as.
Thus remdesivir may begin to resemble oseltamivir an aggressively promoted agent with dubious clinical benefit. Remdesivir in adults with severe COVID-19. Is the first placebo-controlled double-blinded multi-center RCT of remdesivir.
Catalytic Asymmetric Synthesis of the antiCOVID19 Drug Remdesivir Dr. The World Health Organization WHO has characterized this disease as a pandemic. Remdesivir in adults with severe COVID-19.
Remdesivir in adults with severe COVID-19. 2020 there was no limit on the duration of symptoms before study enrolment. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus 2019-nCoV in vitro.
Currently coronavirus disease 2019 COVID-19 is a very serious health problem. Wang Y Zhang D Du G et al. Remdesivir use was not associated with a difference in time to clinical improvement hazard ratio 123 95 CI.
Wang Y Zhang D Du G et al. The primary outcome was time to recovery measured in days after. The study was stopped early due to poor recruitment after including 237 patients but otherwise appears well designed.
Wang D Hu B Hu C et al. Gilead Sciences began accepting requests from clinicians for compassionate use of remdesivir on January 25 2020. The primary outcome was to assess the incidence of AKI and hepatic injury.
A randomised double-blind placebo-controlled multicentre trial. The experimental antiviral drug remdesivir manufactured by Gilead was granted Emergency Use Authorization by the US Food and Drug Administration in May 2020 for patients hospitalized with severe COVID-19. Recommendations related to vaccines and drugs are not available yet because they are still in the clinical trial phase and one of the superior drugs is remdesivir which has an antiviral activity.
Basic Protocol 3 describes the synthesis of remdesivir. One patient in the placebo group who withdrew after randomisation was not included in the ITT population. 30 269271 2020.
Figures and some textual material are taken from our recently published work Wang et al 2020 and rewritten as step-by-step protocols to support reproduction of the original work. 1213 In vitro remdesivir inhibits all human and animal coronaviruses tested to date including SARS-CoV-21315 and has shown antiviral. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus 2019-nCoV in vitro Cell Res.
Between Feb 6 2020 and March 12 2020 237 patients were enrolled and randomly assigned to a treatment group 158 to remdesivir and 79 to placebo. A randomised double-blind placebo-controlled multicentre trial. This is a retrospective chart review study including adult patients admitted with COVID-19 receiving remdesivir with a baseline eGFR 30 mlmin per 173 m2 from December 2020 to May 2021.
Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus 2019-nCoV in vitro. Intriguingly after pretreatment remdesivir slightly enhanced EV71 replication at low concentrations and inhibited virus replication at 100 μM. The first by Wang and colleagues 2.
Unlike a prior study of remdesivir Wang Y et al. 1 As of January 27 2020 the Chinese authorities reported 2835 confirmed cases in mainland China including 81 deaths. SYNTHESIS OF 2-ETHYLBUTYL CHLORO PHENOXYPHOSPHORYL- L -ALANINATE rac -4.
Wang Y Zhang D Du G et al. This work was also supported by the China Evergrande Group Jack Ma Foundation Sino Biopharmaceutical Ping An Insurance Group. Remdesivir Veklury GS-5734 has undoubtedly become an important molecule.
Clinical characteristics of. A randomised double-blind placebo-controlled multicentre trial published correction appears in Lancet. Although we cannot find an explanation for the recent phenomenon the latter inhibition may be because the removal of remdesivir was.
1 To date COVID-19 has led to approximately 23 millions infections and 800 thousand deaths since the end of 2019. The secondary outcome was to assess the efficacy of remdesivir defined by change in oxygen requirement. Wang M Cao R Zhang L et al.
Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus 2019-nCoV in vitro. Xiao Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus 2019-nCoV in vitro. 1 At the time there had been 2 randomized clinical trials RCTs that had compared a 10-day course of remdesivir with placebo.
Remdesivir in adults with severe COVID-19. Wang M Cao R Zhang L. 1569 - 1578.
Wang Y Zhang D Du G et al. Furthermore the numbers and rates of infections and related deaths are increasing with 7. Remdesivir in adults with severe COVID-19.
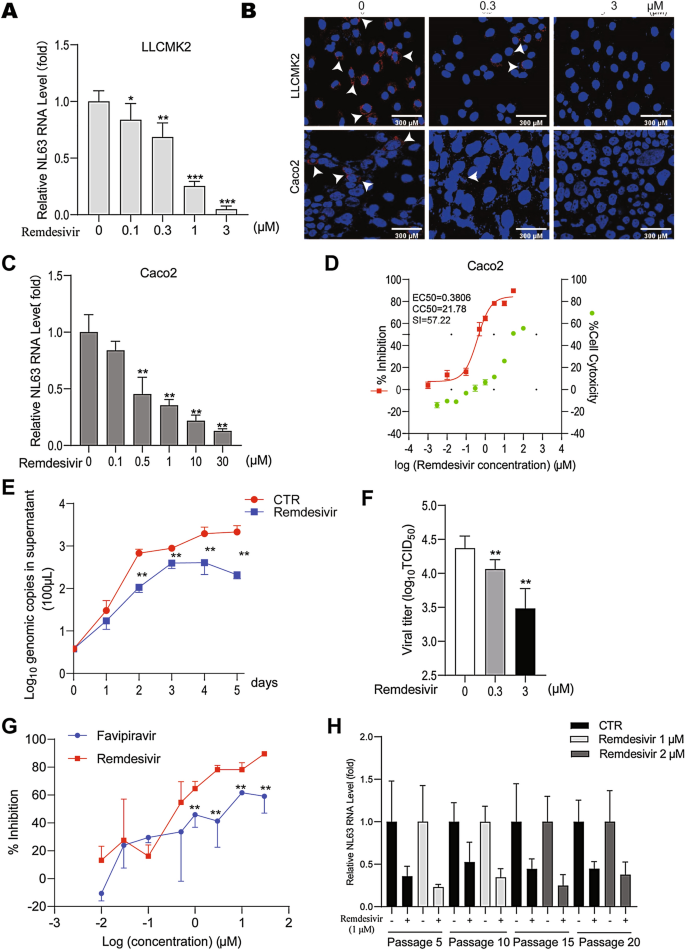
Comparative Assessment Of Favipiravir And Remdesivir Against Human Coronavirus Nl63 In Molecular Docking And Cell Culture Models Scientific Reports
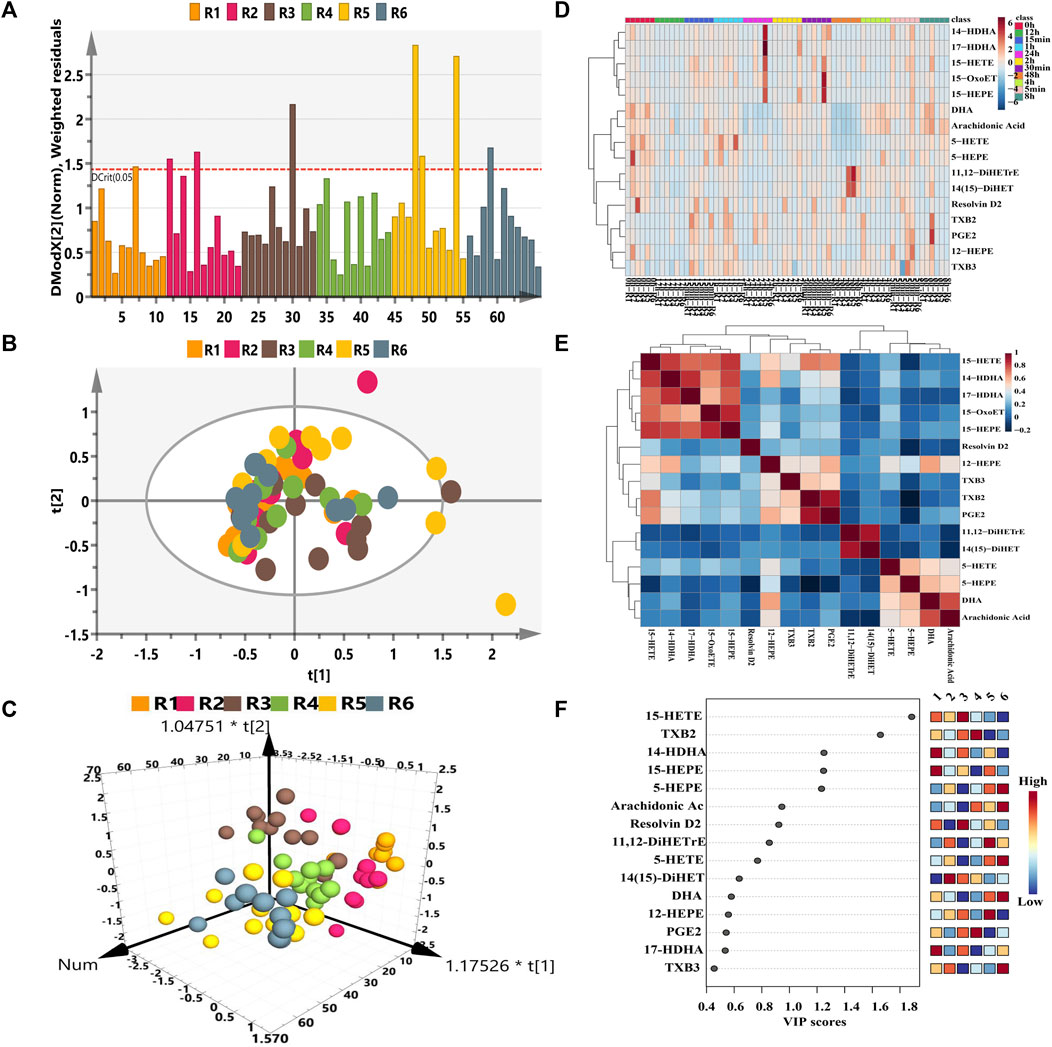
Frontiers Eicosanoid Metabolomic Profile Of Remdesivir Treatment In Rat Plasma By High Performance Liquid Chromatography Mass Spectrometry Pharmacology
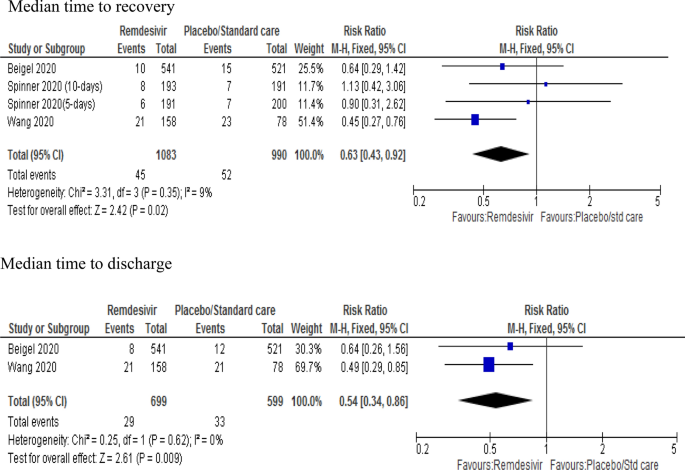
Efficacy And Safety Of Remdesivir In Hospitalised Covid 19 Patients A Systematic Review And Meta Analysis Springerlink

China Begins Testing An Antiviral Drug In Coronavirus Patients The New York Times
Posting Komentar untuk "Wang 2020 Remdesivir"